NIH Proposal Specifics

- Starting your NIH/grants.gov Proposal
- Components of an NIH Proposal
- Significant or Recent Changes
- About Budgets
- Tips for AREA proposals
- Compliance Specifics
- FAQs
- Helpful Information and Links
COVID-19 Information for NIH with COVID-19 FAQs
Starting your NIH/grants.gov Proposal
Proposal preparation and process in brief
-
Starting point
To submit a proposal to the National Institutes of Health (NIH), both the individual and institution need to complete some registrations. Carleton College has alrea dy done the required registrations with Grants.gov and eRA Commons, but each individual principal investigator (PI) must have an account in the NIH online portal of eRA Commons before submitting a proposal (refer to NIH's Investigators and Other Users page, and NIH eRA Password Policy). An individual working in Grants.gov Workspace must also be registered in Grants.gov (see NIH's Grants.gov - User Registration). -
- If you don't yet have an eRA Commons account, call the Grants Office (507-222-4441 or 507-222-4046) for assistance.
-
Proposal preparation via ASSIST or Grants.gov Workspace
-
- Principal Investigator (PI) confers with their program officer at NIH to determine the best program solicitation (PIs from liberal arts colleges often apply to NIH R15 AREA program, but also R01 or R03 and others; see this NIH Plan Your Application page).
- The Grants Office staff or the applicant can go to the ASSIST page and enter the Funding Opportunity Announcement (FOA) number, or to Grants.gov for Workspace, to initiate an application and complete the forms online (use FORMS-F application packages for due dates on or after May 25, 2020), populating forms with standard college information. NIH's newer ASSIST portal is the online system for the preparation, submission, and tracking of grant applications through Grants.gov to NIH. Refer to the Preparing Your Application Using ASSIST page (with helpful Getting Started: Video), the ASSIST User Guide, and ASSIST Online Help.
- An application can be worked on together via ASSIST, the Grants.gov Workspace, or a local Dropbox shared space; or individual pieces for the proposal can be sent to the Grants Office staff who will input them into the online portal. We refer to the guidelines offered in both the application guide AND the specific Funding Opportunity Announcement (FOA). Note that the FAO instructions always supersede application guide instructions.
-
Submission
-
- The SF424 (R&R) proposal application is submitted via grants.gov by designated Authorized Organization Representatives (AOR): Carleton's AORs are the Dean of the College and the Grants Office personnel.
- The NIH retrieves the application from grants.gov and processes it in their eRA Commons online portal, assembling the submitted forms and PDF attachments into a cohesive application.
-
Viewing
The PI and the Grants Office staff login to eRA Commons to see if any “errors” or “warning” notices are identified for your submission. An application must be error-free to complete the electronic submission process. There is a 2-day viewing opportunity before the application moves on to the review process.
A few formatting particulars
(from NIH's How to Apply-Application Guide page, "Format Attachments" under "Write Application")
Fonts
The following typefaces are recommended for text in PDF attachments
- Arial, Georgia, Helvetica, Palatino Linotype.
Other fonts are acceptable if they meet the requirements below.
- Font size: must be 11 points or larger (excepting figures, graphs, diagrams and charts).
- Type density: must be no more than 15 characters per linear inch (including characters and spaces).
- Line spacing: must be no more than six lines per vertical inch.
- Text Color: No restriction (though black or other high-contrast text colors are recommended to be used within grant applications attachments).
PDF File names
- are 50 characters or less (including spaces)
- can use one space (not two or more) between words
- should avoid use of "&"/ampersand
Note: all attachments must be in PDF format.
Margins
- need to be at least 1/2 inch (top, bottom, left, right) for all pages
- NO information should appear in the margins (do not include headers or footers), including PI name or page numbers, as pagination is system-generated
Hyperlinks and URLs
- are only allowed when specifically noted in funding opportunity announcement and form field instructions (e.g., biosketches, publication lists)
- when allowed, hyperlink the actual URL text rather than hiding the URL behind a specific word or phrase
- reviewers are not obligated to view linked sites; don’t use hyperlinks anywhere else in your application
Components of an NIH proposal
Mandatory forms for many NIH proposals, with commonly used attachments (in bold italics)
-
SF424 (R&R)
This Research & Related (R&R) form is where basic information is identified, such as type of submission, institution's name/DUNS/contacts, PI contact information, title and ask amount. -
The Cover Letter Attachment is attached to this form. See below under "Optional Pieces."
-
PHS 398 Cover Page Supplement
An additional form where we identify such aspects as vertebrate animal practices, program income information, or inventions and patents. Details are at G.210-PHS 398 Cover Page Supplement Form.
-
R&R Other Project Information
-
- Project Summary/Abstract
-
- no more than 30 lines of text
- give a succinct and accurate description of the proposed work that states broad objectives and specific aims with reference to the health relatedness of project
- Project Narrative
-
- no more than 2-3 sentences
- describe relevance to public health
- Bibliography & References Cited
-
- no page limitation
- provide bibliographic citations based on the Research Plan, including names of all authors
- note the Public Access Policy for NIH supported pubs requires specific citation (need to provide the NIH Manuscript Submission reference number)
- Facilities and Other Resources
-
- no page limitation
- describe resources available, and address specific program requirements (i.e., R15/AREA applications ask for specifics such as a profile of students, description of how the scientific environment will contribute to the probability of success of the project, how the project will benefit from unique features of the environment
- must include a plan for how RCR training will be conducted
- Early Stage Investigators need to explain the institutional investment in the success of the investigator, e.g., resources, classes, etc.)
- Equipment
-
- no page limitation
- list major items of equipment already available for the project
-
Project Performance Site Location(s)
The primary project location (or locations) is identified on this form.
-
R&R Senior/Key Person Profile
-
- Biographical Sketch(es): maximum of 5 pages, required for each individual identified as key personnel; provide text for sections of
-
- A. Personal Statement
- B. Positions and Honors
- C. Contribution to Science
- D. Additional Information: Research Support and/or Scholastic Performance
-
PHS 398 Research Plan
Pieces most often needed for this form are listed below. Additional attachments may include Letters of Support, Vertebrate Animals, Multiple PD/PI Leadership Plan, or sections for human subjects. Refer to the Research Plan Section of the Forms Version F General (G) Instructions for the SF424 (R&R). See "Optional Pieces" below for more on vertebrate animals. -
- Specific Aims
-
- 1 page
- address research goals, expected outcomes, impacts
See Specific Aims -
- Refer to the FAQ below "What is the recommended structure for the Specific Aims page?"
- NIH personnel state that the abstract and specific aims pages are "most read so make sure these are strong, clear, significant, and compelling" and they suggest that PIs get feedback on this 1-page Specific Aims.
- Research Strategy
-
- limited to 12 pages for R15/AREA (see newest R15 solicitation PAR-18-714 clinical trial not allowed) and for RO1s
- describe the project, addressing specific sections of Significance, Innovation, Approach
See 3. Research Strategy
-
- Enhancing Rigor and Reproducibility Resource chart provides a guide to how and where in the Research Strategy document an applicant should address four key "areas of focus" in the application (see also this Guidance: Rigor and Reproducibility page:
-
- Rigor of the Prior Research - "Significance" and "Approach" section
- Scientific Rigor (Design) - "Approach" section
-
- Note that NIH research shows that the quality of this section is the most important predictor of whether a given application receives funding.
- Biological Variables - "Approach" section
- Authentication - As a separate attachment, not in the Research Strategy
- Resource Sharing Plan
-
- 1 paragraph
- data sharing applies to applications with direct costs of $500,000 or greater in any single year (so not often applicable to us)
- may also possibly need to address Sharing Model Organisms or Genomic Data Sharing
See Resource Sharing Plan(s)
In addition, applicants can obtain more general advice on resource sharing on the Grants Office's Dissemination and Sharing of Research Results page.
Optional pieces
Budget
A budget is ALWAYS REQUIRED, but NIH labels the budget forms as "Optional" in the application portal or package, due to applicants being asked to choose from either
- a modular budget or
- a detailed Research & Related (R&R) Budget
Learn of the distinctions between the two types below in the About Budgets section.
-
Round the budget numbers: “While the dollar fields allow cents to be entered, all dollar fields should be presented in whole numbers. Please round to the nearest whole number.”
Cover Letter Attachment
Though optional, a cover letter is encouraged
- to provide explanations for a late application or subaward budget component,
- for inclusion of a video,
- if planning to generate large-scale either human or nonhuman genomic data as part of the study,
- if preapproval is required, or
- for other unusual situations.
See Cover Letter Attachment. Note that this attachment must NOT be used to communicate application assignment preferences.
A resource for helping determine whether to include a cover letter and what to include is “Cover Letters and Their Appropriate Use” (MP3, Transcript).
PHS Assignment Request Form
an optional form - the PHS Assignment Request form (starting May 25, 2016) - that includes information previously collected in the "Cover Letter Attachment," gives the PI an opportunity to indicate
- awarding component assignment preference,
- study section assignment(s) request,
- individuals who should not review your application due to conflicts, and
- specific areas of expertise needed to review your application.
For Vertebrate Animals
If vertebrate animals are involved: as a part of the Research Plan Form, address the criteria identified in the Vertebrate Animal section (no page limit)
For additional information, see this Worksheet for Applications Involving Animals.
If YES to Vertebrate Animals even if the review/approval process has not yet begun, say "Yes" to "Is the IACUC review Pending?"
Call the Grants Office (x4441) for Carleton's Animal Welfare Assurance number.
For more:
- view a podcast on "Writing Your Vertebrate Animal Section" (on this "All About Grants Podcasts" page you can choose between MP3 or Transcript)
- refer to requirements in the Public Health Service (PHS) Policy: PHS Policy HTML Version and PHS Policy PDF Version. The PHS Policy is summarized in the brochure What Investigators Need to Know About the Use of Animals. Additional information can be found at the Office of Laboratory Animal Welfare.
Multiple Investigators or Consortiums
Collaborative proposals are those in which investigators from two or more organizations wish to collaborate on a unified research project.
If your project involves multiple investigators, talk with the Grants Office as there are special considerations
- in form completion and budgeting calculations (examples: a Multiple PI/PD Plan is to be included within the Research Plan Section
- if there are consortium/contractual activities they are to be described and attached per guidelines in section #12 of the Other Research Plan Section
- if a subaward is proposed, instructions for preparing the budget form are at R&R Subaward Attachment(s) Form
Significant or Recent Changes
R15/AREA changes
Starting in January 2019, NIH administers two programs using the R15 activity code:
- the Academic Research Enhancement Award (AREA) targeting undergraduate-focused institutions, that requires a letter of eligibility (see Sample Provost Letters Certifying Eligibility). AREA has two programs:
-
- (PAR-18-714) Clinical Trial is Not Allowed
- (PAR-19-133) Clinical Trial is Required
- the Research Enhancement Award Program (REAP) targeting graduate schools of arts and sciences and health professional schools that grant baccalaureate or advanced degrees (PAR-19-134)
For more: check R15 Frequently Asked Questions and NIH Research Enhancement Award (R15) webpage
Forms Version F for use starting May 25, 2020
Applicants must use FORMS-F application packages for due dates on or after May 25, 2020 and must use FORMS-E application packages for due dates on or before May 24, 2020. FORMS F, or SF424 (R&R) - Version F Instructions are found on the How to Apply - Application Guide page.
Refer to the Significant Changes section of the Version F instructions, and to NOT-OD-20-077.
Additional resources: NIH FORMS-F Application Forms Update (video, 8 min), and this Annotated Form Set for NIH Grant Applications: FORMS-F Series.
Other Updates
Biographical Sketch
This biosketch instructions notice gives clarification and consolidated biosketch instructions for application with due dates on or after May 25, 2016 and continuing.
Changes since 2016 include:
- Indicate that an URL for a publication list is optional and, if provided, must be to a government website (.gov) like My Bibliography.
- Allow publications (peer-reviewed and non-peer-reviewed) and research products to be cited in both the personal statement and the contributions to science sections
- State that graphics, figures, and tables are not allowed.
- Remove the requirement that the past 3 years of research support are listed in order of relevance.
- Give option to add other names used to author research products in section A.
- Allow research products to include conference proceedings such as meeting abstracts, posters, or other presentations.
- Allow research products that are under development, such as manuscripts that have not yet been accepted for publication, to be mentioned in the narrative sections; however, they cannot be cited as one of their citations.
If Vertebrate Animals are involved
Some changes for applications submitted for due dates on or after January 25, 2016 and continuing (see NOT-OD-16-006):
- A description of veterinary care is no longer required.
- Justification for the number of animals has been eliminated.
- A description of the method of euthanasia is required only if the method is not consistent with AVMA guidelines.
Other changes starting in 2016 and continuing
Title: On a much smaller scale, the title can now be 200 characters (with spaces) rather than the previous 81 character limit.
PDF file names: pdf file names can use a space to separate words rather than underscore.
Starting in May 25, 2016 and continuing
- New, optional "PHS Assignment Request Form" that complements existing "Cover Letter Attachment" on SF424 (R&R) form and gives PI opportunity to indicate: funding component assignment preference, individuals who should not review your application due to conflicts, and scientific areas of expertise needed to review your application.
- PHS 398 Cover Page Supplement Form changes include a new Vertebrate Animals section, removal of the "Disclosure Permission Statement" question, and other small changes or updates.
About Budgets
Budget & Budget Justification
MODULAR budget
1) A modular budget is used if direct costs are $250,000 or less per budget period (and all requested under Period 1 if submitting an R15/AREA proposal). A budget period is typically one year of support, though R15/AREA grants are an exception.
Refer to the Cumulative Budget Information section, Modular Budget Justifications (of Personnel Justification, Consortium Justification, or Additional Narrative Justification). No page limitations.
RESEARCH & RELATED (R&R) budget
2) If asking for $250,001 or more in direct costs, use a Research & Related (R&R) more detailed budget. (Again, if submitting to R15/AREA, the total budget for all years of the proposed project must be requested in Budget Period 1.) Use R&R Budget item K attachment for budget justification -address both Personnel Justification & student involvement. No page limitations.
Include a Budget Justification for an R&R budget to provide additional information requested in each budget category identified. Items and amounts need to be considered necessary, reasonable, allocable, and allowable.
Preparing your NIH budget
There are specific requirements for R15/AREA submissions (discussion of student involvement, how numbers are inputted). Talk with the Grants Office and refer to the R15/AREA (Academic Research Enhancement Award) for Undergraduate-Focused Institutions program announcement PAR-18-714.
To access a budget template spreadsheet for internal use, go to the the Grants Office Forms & Templates page.
For current figures to use in budget preparation - such as Carleton College faculty and student compensation guidelines, benefit percentages, indirect cost rate, and more - contact the Grants Office (Dee 507-222-4441, Charlotte 507-222-4046, or Christopher 507-222-5833).
INDIRECT COST is a line item in nearly every proposal budget submitted to a federal agency. In contrast to straightforward project expenses of "direct costs" (such as salary, benefits, equipment, travel, supplies), indirect costs - also referred to as F&A (Facilities & Administrative Costs) - are "those costs which are not readily identifiable with a particular cost objective but nevertheless are necessary to the general operation of an organization."
An Indirect Cost Rate (IDC) agreement is negotiated with a Federal agency every four years. Carleton's IDC rate agreement, negotiated with DHHS 4/22/19 based on our Audited Financial Statements, has been approved at a rate of
60% (of salaries and wages) for federal grants with award dates of July 1, 2019 through June 30, 2023.
The Business Office government grant proposal page explains that the rate is "calculated on the total of all faculty or technician salaries/stipends and undergraduate student stipends."
The indirect cost rate in effect at the time of the initial awarding of a grant is in effect throughout the life of the grant.
EFFORT is recorded in any budget, and expressed in person months; see the FAQ (below) "What are person months and how do I calculate them?"
Tips for AREA proposals
Program officers for NIH's AREA (Academic Research Enhancement Award) program, clinical trial not allowed (PAR-18-714) offer these tips for successful proposals:
Be mindful of a key criterion
A key criterion for AREA projects is how the grant will improve the "research environment" at the institution, primarily by expanding programmatic or administrative capacities related to research. Too many proposals neglect this criterion.
Consider including a diversity supplement
AREA grants can include, or subsequently seek, "diversity supplements" to support outreach from the institution to K-12 students, or even undergrad/grad students at other institutions. These awards are purported to be easy to request and obtain - especially if the supplement is used to continue to employ students from underrepresented groups as researchers in the second half of the grant period.
Dovetail biosketch info with the Facilities doc
The biosketch should dovetail well with the "Facilities and Other Resources" document on the institution itself - e.g., internal grant awards listed in the biosketch should be described in the resource area as faculty-development programs.
Phrase scientific aims as hypotheses
The scientific aims are best phrased as questions/hypotheses, not statements. These questions can then be recycled in grant reports, which will provide answers to the questions.
Use a graphic to match aims with resources/personnel
PDs should use a graphic (timeline/table/chart) to lay out their aims and match those aims to the resources - especially the personnel, including the PD and undergrad researchers (for whom continuity across the project can be a question).
Reflect on these pieces of advice from PUI PIs
- Involve first years and sophomores, require a multi-semester commitment, and involve students in training new students
- Given academic year fluctuations, consider a technician (part time or seasonal), duties for existing employees (teaching, lab maintenance), whether a shared tech for several labs/departments might be of benefit?
- In the Research Strategy, address project feasibility with involvement of students
- Teach experimental design in your lab classes
- Consider cultural exposure to major research institutions
- Assess what students need to participate (e.g., course credit, hourly pay, summer wages)
Compliance specifics
What do I need to do to be compliant with federal requirements?
Each organization receiving funding from a federal agency needs to certify that the institution and individuals are following specified federal guidelines. Carleton asks all primary investigators (PI and coPIs) involved in a proposal to a federal governmental agency (NSF, NIH, NEH, etc.) to read and sign a Compliance and Disclosure form (done via this Link to OnBase Form that requires OnBase login to access the form). The form addresses college policies and provides a checklist with links addressing I) financial conflicts of interest (FCOI), II) human or animal subject involvement, III) responsible conduct of research, and IV) environmental health and safety issues.
For FCOI, per the NIH Grants Policy Statement, institutions need to require investigators to complete the training prior to engaging in NIH-supported research and at least every four years, and when an investigator is new to an institution, or if an investigator is noncompliant with Carleton's FCOI policy. Completing this NIH FCOI interactive training module satisfies the regulations.
What is RCR and how do I comply?
RCR stands for "responsible and ethical conduct of research." The submitting organization must certify that there is "a plan to provide appropriate training and oversight in the responsible and ethical conduct of research to undergraduate students, graduate students, and postdoctoral researchers participating in the proposed research project." NIH states that the "grant application must include a plan for how you will carry out instruction in responsible conduct of research."
Go to the the Grants Office page Responsible and Ethical Conduct of Research to link to CITI online training modules and to obtain more specific information on online RCR instruction.
See NIH's Notice (NOT-OD-10-019) Update on the Requirement for Instruction in the Responsible Conduct of Research and Policy on Instruction in the Responsible Conduct of Research (pdf pg 337, specific to Career Development Grants).
FAQs
What is the definition of a clinical trial?
A clinical trial is "a research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes."
How do I determine which NIH Institute or Study Section is the best fit for my project?
- Pre-submission:
-
- Use NIH RePORTER's nifty Matchmaker tool (with helpful short tutorial) to find which NIH Institutes and Centers have funded similar projects and to access contact information for Program Officials (PO) or Program Directors (PD). This Using RePORT to Your Advantage presentation gives detailed instructions and screen shots.
- Contact the PO/PD of an NIH Institute that seems to most closely align with your research. The PO/PD, specific to a particular institute, manages a scientific research portfolio and can help you determine a fit (if not his/her institute, they can point you to a different one). Ask if the PO is willing to read a brief outline of the proposed project or draft of the specific aims.
- Post submission:
-
- Shortly after submission, questions can be addressed to the SRO (Scientific Review Officer) who helps ensure that your project is fitted to the most appropriate Scientific Review study section. The SRO also recruits qualified reviewers, assigns applications to reviewers, and prepares summary statements for applications reviewed.
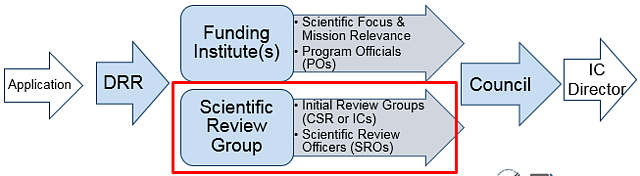
- Shortly after submission, questions can be addressed to the SRO (Scientific Review Officer) who helps ensure that your project is fitted to the most appropriate Scientific Review study section. The SRO also recruits qualified reviewers, assigns applications to reviewers, and prepares summary statements for applications reviewed.
How long from submission until an award is made?
The NIH grants process can take approximately 10 months from application receipt and the peer review process through negotiation and award.
- The Grants Process Overview page includes a resource for understanding the steps your application goes through in that time frame.
- This NIH Peer Review Process: An Overview presentation explains the review process, reveals what reviewers look for in a good proposal, and describes NIH's scoring system. Also, refer to this Peer Review page. After peer review, the assigned PO/PD is available to address questions about the review.
What is the recommended structure for a Specific Aim?
It's been said that the Specific Aims document is the single most important piece of your proposal. For each specific aim, the following structure is recommended:
- Introductory paragraph
-
- What is the research area? What is known? What is the gap in knowledge? What is the critical need? Who cares?
- Second paragraph
-
- What is the solution? What is the long-term goal and potential impact?
- Aims
-
- What will you do to test the hypothesis? What are the expected outcomes?
- Final paragraph
-
- Return to impact/payoff
Am I an early stage investigator?
An individual who - is classified as a New Investigator (has not previously competed successfully as PD/PI for a substantial NIH independent research award), and is within 10 years of completing his/her terminal research degree or is within 10 years of completing medical residency (or the equivalent) - is considered an Early Stage Investigator (ESI). See also the definition of New Investigator (similar to early stage investigator but without the within-10-years-of-completing component) and New and Early Stage Investigator Policies.
What are person months and how do I calculate them?
What is the definition of "person-months"?
"The term "person-months" refers to the effort (amount of time) that PI/coPI(s), faculty, and other senior personnel will devote to a specific project. The effort is based on the type of appointment of the individual with the organization: academic-year (AY), summer term (SM), or calendar-year (CY). For example, if the regular schedule is 10 months and 30% effort will be devoted to the project, a total of 3 months should be listed in the academic or calendar-year block (10 months x 30% = 3 months)." See other approaches below.
How do I calculate the person-months per year committed to the project?
"Multiply the percentage of your effort associated with the project times the number of months of your appointment (i.e., 10% of a 9 month AY appointment equals 0.9 person months; 10% of a 12 month calendar appointment equals 1.2 months)... Person months shown in the current and pending support section should usually equal the number of months on the NSF proposal budget." OR, if you know the number of hours, days, or weeks to be devoted to the project, person-months can be obtained by calculating the portion. For example, working 5 days on a project = 1 week/4 total weeks in a month = 0.25 person-months. Since a month includes a working day or two more than four weeks, an alternate way to calculate would be 5 days/22 work days in a month = 0.23 person-months. Simply said
- Using weeks: multiply number of weeks by 0.23 to get person months (3 weeks x 0.23 = 0.69 person months). OR
- Using days: multiply number of days by 0.05 to get person months (4 days x 0.05 = 0.20 person months).
If the time varies in each year, calculate yearly person months and then average them for the final number to report on the NSF Current and Pending form. If devoting a term to research, the academic year person months can be calculated using 1/3 of 9-month appointment = 3.0 academic months (1/3 for Carleton's trimester system). With the NIH guidelines are different from NSF for summer work: it is permissible to ask for up to 3.0 months with NIH. More on Person Months on this NIH FAQ page.
What is a "Resource Sharing Plan"?
NIH defines 3 types: Data Sharing Plan, Sharing Model Organisms, Genomic Data Sharing. Most often only the Data Sharing Plan applies to an undergraduate institution (though certainly there are exceptions). Data Sharing Plan: Specific funding opportunity announcements may require a plan regardless of ask amount, but if not specified, investigators seeking $500,000 or more in direct costs in any year are expected to include a brief 1-paragraph description of how final research data will be shared, or explain why data-sharing is not possible. For more, see Resource Sharing Plan(s).
Helpful Information and Links
- Scan NIH's Grants Process Overview
- Explore NIH's Write Your Application
- Refer to NIH's Application Guide
- Read through the ppt presentation eRA Session: Ready! Set! Submit! Application Preparation & Submission
- Use RePORTER's nifty Matchmaker tool
- Listen to informative podcasts at NIH's “All About Grants” (transcripts available also)
- Peruse the AREA (Academic Research Enhancement Award)/R15 program announcement/guidelines PAR-18-714 and the NIH AREA Program web page
- Read about how to use the application instructions for Forms Version F
- Watch Grants Administration Take 10: NIH FORMS-F Application Forms Update video
- Refer this Annotated Form Set for NIH Grant Applications: FORMS-F Series
- Learn of and use updated format pages
- Find instructions for completing forms in the Annotated Form Set for NIH Grant Applications - FORMS-F Series
- Read the Research Instructions for NIH and Other PHS Agencies Forms F
- See NIH’s standard due dates
- Review Avoiding Common Errors, Frequently Asked Questions, Tips for the Principal Investigator, eRA Commons Help Desk Top Ten FAQs
- Explore the eRA Home page, with an Applicants tab that provides information on how to use eRA systems pre and post award, a Register/Accounts tab with instructions on how to create and manage accounts, and a Help & Tutorials tab with eRA tutorial videos
- Reference, as necessary, the NIH Grants Policy Statement (updated Oct 2018) accessed via the Policy & Compliance page, and for updates refer to Significant Changes since Oct 2017 or Guide Notices
- Watch some Proposal Writing Tutorials on YouTube
- Learn more about navigating Grants.gov: Applicant Training resources on Grants.gov
- In a 2016 post to the NIH’s “Open Mike” blog, Dr. Michael Lauer, the Deputy Director for Extramural Research, summarized a massive analysis of 123,000 different applications to the flagship R01 program that sought to correlate peer reviewers’ score of various application components (significance, investigator(s), innovation, approach, and environment) with whether the application received funding. In brief, the analysis found that:
-
- by far an application’s approach score, and to a lesser extent, the significance score, were the most important predictors of overall impact score and of whether any given application is funded. What does this mean for you as applicants? We think it’s helpful for R01 applicants to know that the description of the experimental approach is the most important predictor of funding, followed by the significance of the study.
